1.3.4 Bake-out
To achieve pressures in the ultra-high vacuum range (<10
-8 hPa) the following conditions must be met:
- The base pressure of the vacuum pump should be a factor of 10 lower than the required ultimate pressure.
- The materials used for the vacuum chamber and components must be optimized for minimum outgassing and have an appropriate surface finish grade.
- Metallic seals (e. g. CF flange connections or Helicoflex seals for ISO flange standards) should be used.
- Clean work is a must for ultra-high vacuum, i. e. all parts must be thoroughly cleaned before installation and must be installed with grease-free gloves.
- The equipment and high vacuum pump must be baked out.
- Leaks must be avoided and eliminated prior to activating the heater. A helium leak detectors or a quadrupole mass spectrometer must be used for this purpose.
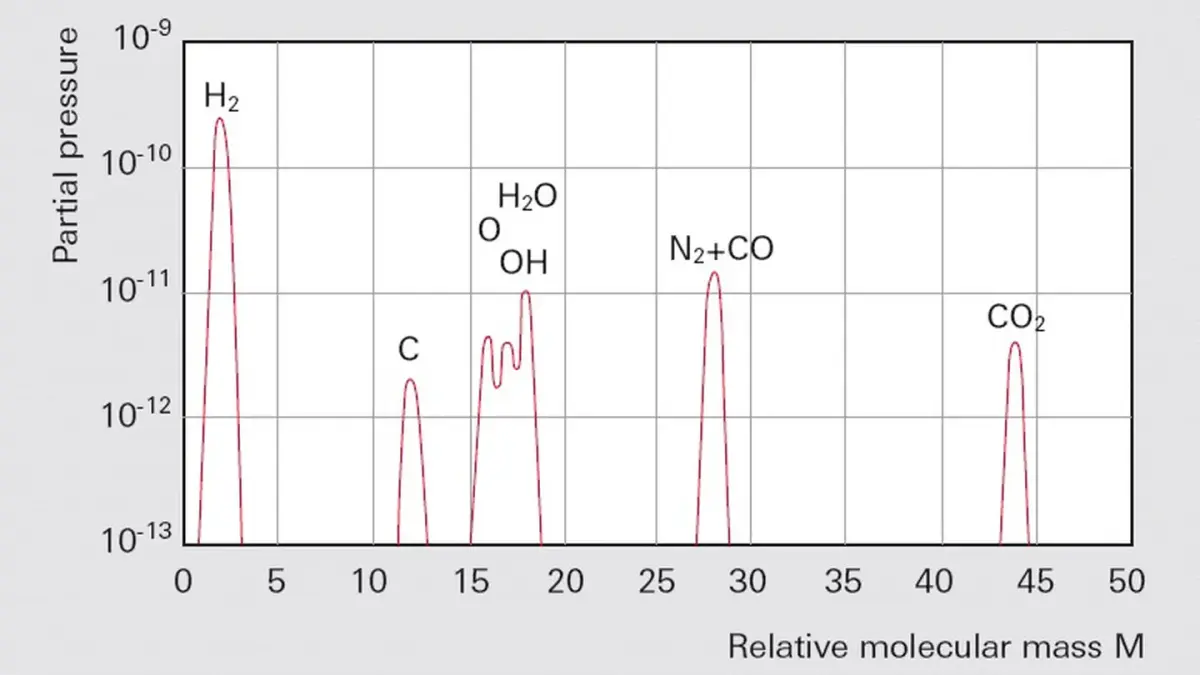
Bake-out significantly increases desorption and diffusion rates, and this produces significantly shorter pumping times. As one of the last steps in the manufacturing process, chambers for UHV use can be annealed at temperatures of up to 900 °C. Subsequent bake-out temperatures may reach up to 300 °C in tents. Pump manufacturers' instructions relating to maximum bake-out temperatures in the high vacuum pump flange normally restrict the maximum temperature during operation to 120 °C. If heat sources are used in the vacuum equipment (e. g. radiation heating), then the admissible radiated power must not be exceeded.
The equipment is put into operation after it has been installed. After reaching a pressure of 10
-5 hPa the heater is switched on. During the heating process, all vacuum gauges must be operated and degassed at intervals of 10 hours. If stainless steel vessels with an appropriate surface finish grade and metal seals are used, bake-out temperatures of 120°C and heating times of approximately 48 hours are sufficient for advancing into the pressure range of 10
-10 hPa.
Bake-out should be continued until 100 times the expected ultimate pressure is attained. The heaters for the pump and vacuum chamber are then switched off. After cool-down, the desired ultimate pressure will probably be achieved. At pressures of less than 5 · 10
-10 hPa and large interior surface areas, it will be advantageous to use a gas-binding pump (titanium sublimation pump) that pumps the hydrogen escaping from the metals at a high volume flow rate.
_grayscale(false)_format(webp)_news_article_1200x675.webp)