Why Container Closure Integrity Testing (CCIT) matters more than ever
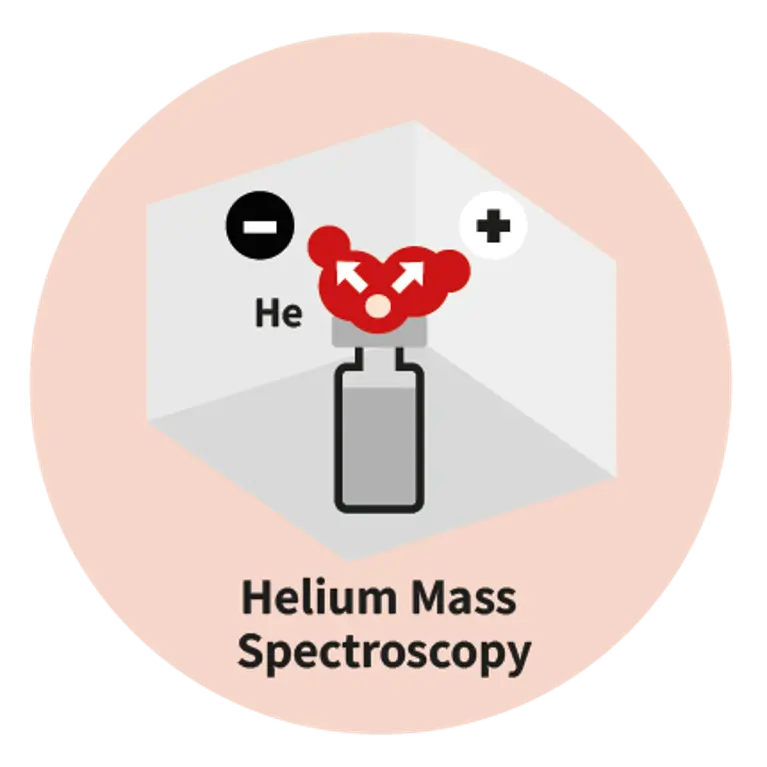
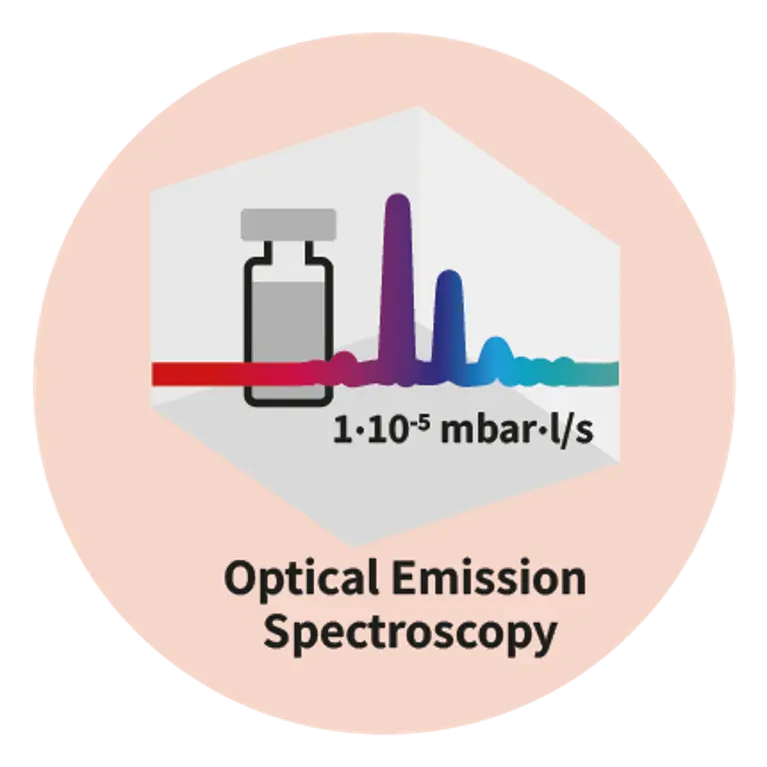
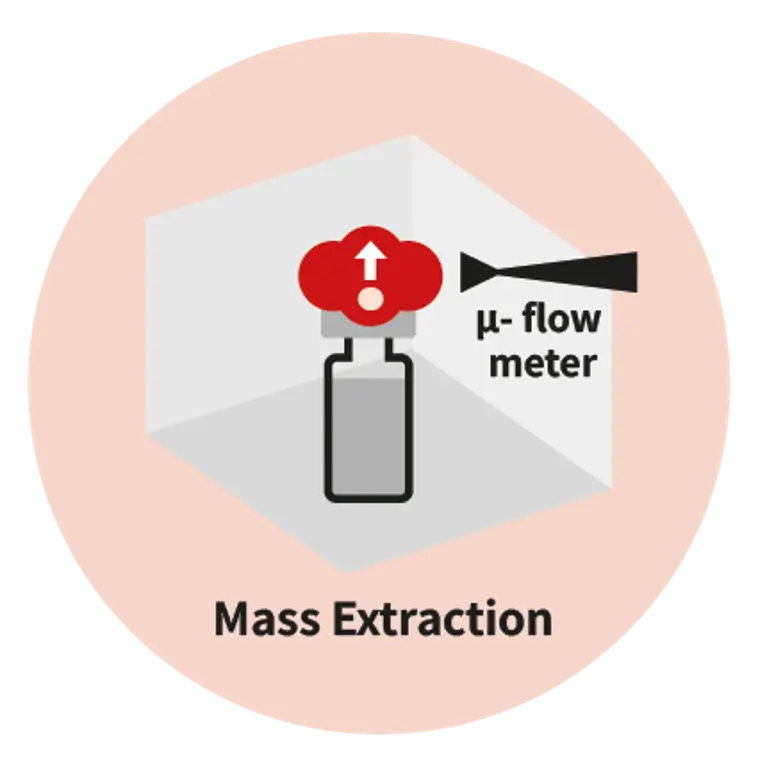
As drug delivery systems become more complex, so does the need for precision in packaging. Whether you are working with pre-filled syringes, vials, auto-injectors, or IV (intravenous) bags, any micro-defect in the container and closure system can compromise sterility, patient safety, and regulatory compliance. Traditional leak tests like blue dye or microbial ingress have proven insufficient, often missing defects smaller than 10 µm. That is why regulators are pushing for deterministic, non-destructive test methods, and why leading pharma as well as biotech companies are making the shift today.Pfeiffer offers expertise in three advanced technologies: helium mass spectrometry, mass extraction and optical emission spectroscopy, providing tailored solutions to meet stringent pharmaceutical and medical demands.