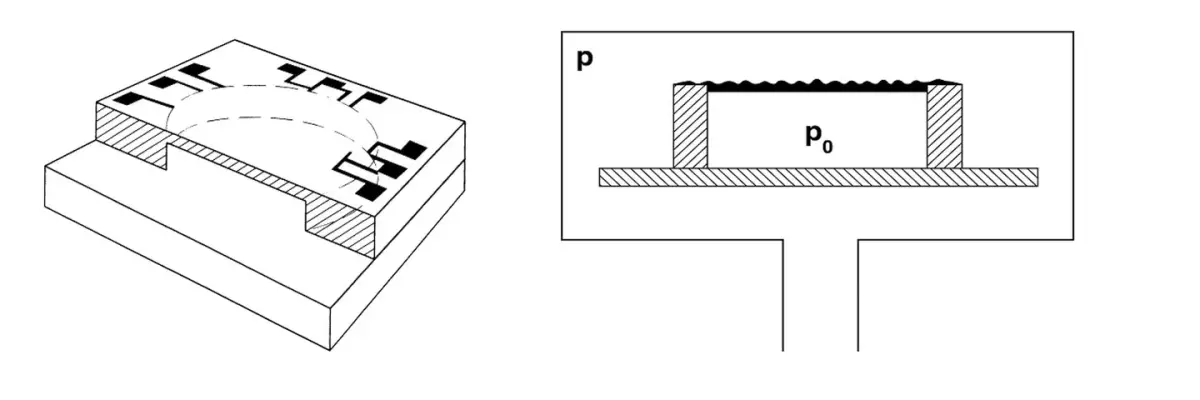
5.1 Fundamentals of total pressure measurement
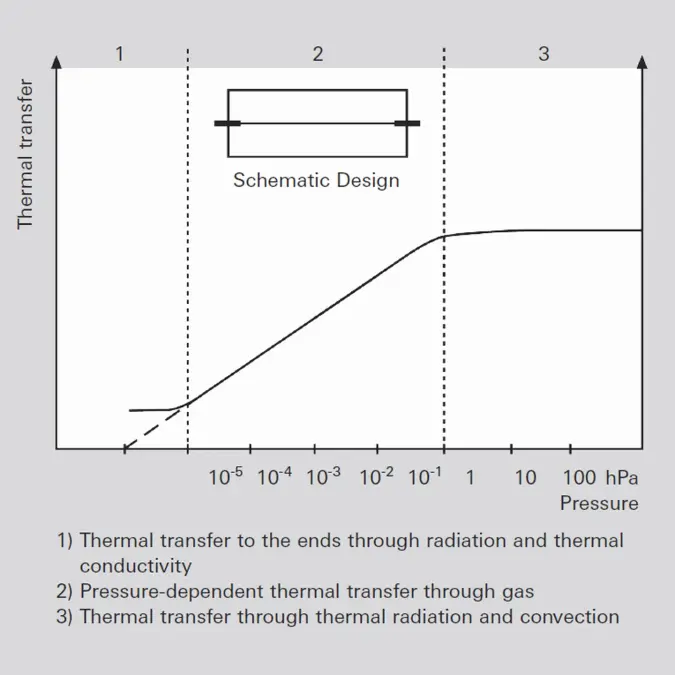
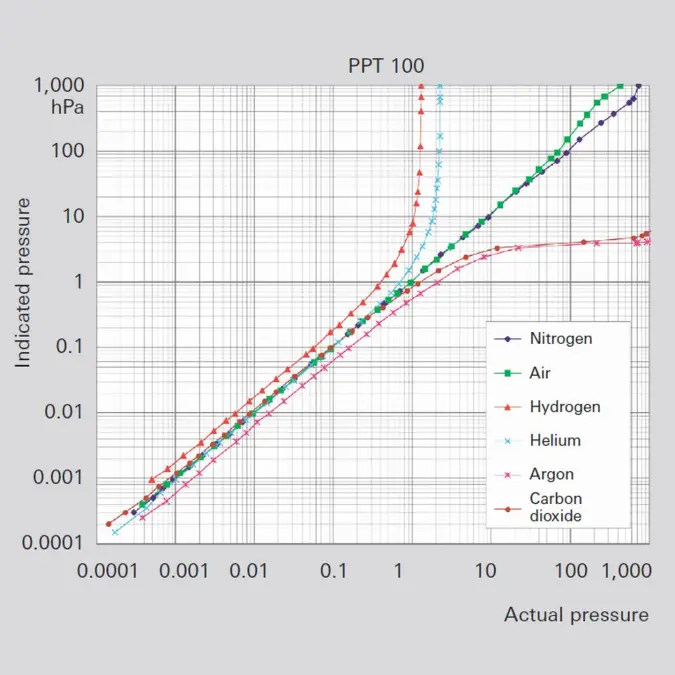
Pressure is defined as force per unit of area: p=F/A (Formula 1-3) where F is the force and A the area to which the force is applied. The SI unit of pressure is 1 N / m² = 1 Pa. Other frequently-used units of pressure are: 1 mbar = 1 hPa = 100 Pa and 1 Torr = 133.322 Pa. If pressure is measured via the force that is exerted on an area, the pressure measurement is independent of the type of gas.Pressure measurement on the basis of force reaches its limits at pressures of less than 1 hPa, because the exerted forces become too small. Consequently other processes must be used. The thermal conductivity of the enclosed gas can be used, for example, or the gas molecules can be ionized and the ion current flowing between electrodes measured. These indirect measurements which determine the pressure from a gas property consequently deliver a measurement result that is dependent on the type of gas.
In vacuum technology, no single measurement method covers the entire pressure range. It is therefore necessary to use different sensors. The criteria for selecting a pressure sensor are based upon various conditions:
- The pressure range to be detected
- Gas composition: Inert or corrosive
- Required accuracy and repeatability
- Environmental conditions, such as radioactivity